Most gecko species reproduce sexually and both a male and female are required for successful breeding. But how are the sexes produced? How does a gecko egg “know” to produce a male or female hatchling? Answering these questions depends upon which species of gecko you are talking about, because geckos have come up with a variety of ways to determine sex.
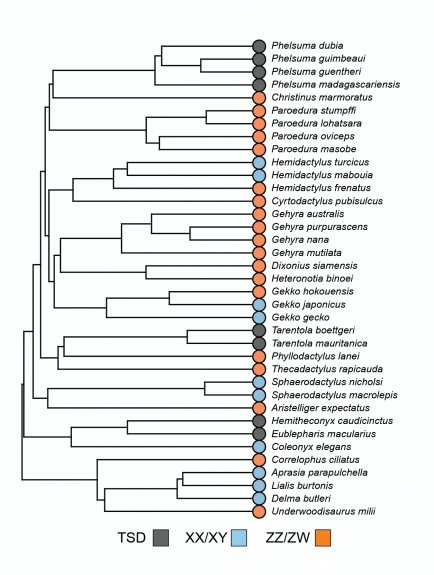
Gecko Sex Determination
The process that directs an embryo to become a male or female is called sex determination, which occurs while the embryo is still in the egg during the middle third of incubation. Most of us are familiar with how humans and other mammals determine sex using a sex chromosome system where males have an X and Y chromosome while females have two X’s. This is called an XX/XY sex chromosome system. Some gecko species, like the Mediterranean House Gecko (Hemidactylus turcicus), the Central American Banded Gecko (Coleonyx elegans), and Burton’s Legless Lizard (Lialis burtonis), have this sort of sex-determining system too. Some other gecko species have a different kind of sex chromosome system called a ZZ/ZW system. Here, the female has two different sex chromosomes called a Z and W while the male has two Z chromosomes. Geckos like the Common House Gecko (Hemidactylus frenatus), Turnip-tailed Gecko (Thecadactylus rapicauda), and Crested gecko (Correlophus ciliatus) have a ZZ/ZW sex chromosome system. In species with sex chromosomes the sex of the embryo is determined at fertilization depending on its chromosome complement. Most of the time this results in males and females hatching at an approximately even 1:1 sex ratio, half the hatchlings should be male while the other half are female.

Some gecko species don’t have sex chromosomes at all and sex is instead determined by incubation temperature. Leopard Geckos (Eublepharis macularius), African Fat-tailed Geckos (Hemitheconyx caudicinctus), and Crocodile Geckos (Tarentola mauritanica) all have temperature-dependent sex determination or TSD. Some incubation temperatures produce mostly males, others mostly females, and still other temperatures, often in a very narrow range, will produce a mix of both sexes. Exact temperatures and patterns vary by species.
The Sliding Scale
Thinking about sex-determining modes as two, discreet categories: (1) genetic system using sex chromosomes; and (2) environmental systems using TSD; may not reflect the true diversity of sex-determining mechanisms. There is emerging evidence that sex-determining systems may be better thought of as a gradient, with sex chromosomes and TSD at extreme ends of a spectrum. Some of this evidence comes from studies of Schlegel’s Japanese Gecko (Gekko japonicus). Karyotypes (viewing chromosomes under a light microscope) indicate this species has an XX/XY sex chromosome system although incubation experiments suggest temperature can influence hatchling sex ratio. Can sex chromosomes and TSD co-exist in the same species? While we don’t have a definitive answer yet in geckos, recent research involving Central Bearded Dragons (Pogona vitticeps) has been illuminating. Bearded dragons have a ZZ/ZW sex chromosome system and researchers at the University of Canberra in Australia have developed a simple test to identify W chromosome DNA to determine the genetic sex of an individual. Sex ratios at most temperatures are 1:1 but things get strange as the incubation temperature rises. At high incubation temperatures, all of the hatchlings are females. However, genetic tests indicate that only half of those high temperature females have W chromosomes. In other words, half of those females are sex-reversed with ZZ chromosomes! Even more interesting, when you breed a (normal) ZZ male to a ZZ sex-reversed female the sex of their offspring is determined not by sex chromosomes (none of these adults has a W chromosome anymore) but is instead determined by incubation temperature. These researchers took a species with ZZ/ZW sex determination changed it to TSD in a single generation! They have subsequently found a ZZ female in the wild indicating this is not an artifact of captivity. The Bearded Dragon studies highlight the extreme plasticity of reptile sex-determining systems and suggests we have much more to learn.
Parthenogenesis
While geckos have an extraordinary diversity in sex-determining mode, some species have done away with sex altogether. These are parthenogenetic species and are the result of two closely related species hybridizing. The hybrid offspring are all female and clone themselves. This includes Bynoe’s Gecko (Heteronotia binoei), Mourning Geckos (Lepidodactylus lugubris), and IndoPacific Geckos (Hemidactylus garnotii), among others. It is important to note that these gecko species are obligate parthenogens and can only reproduce asexually. This is different from facultative parthenogenesis, which has been documented in some species of sexually reproducing snakes and lizards and occurs when a female has fertile offspring without mating.

The Remarkable Diversity of Geckos
Geckos are remarkable because they have multiple modes of sex-determination. However, what makes geckos truly extraordinary is that these different sex-determining mechanisms have evolved repeatedly in different species. Few other reptiles, indeed, few other vertebrates, are as diverse as geckos when it comes to sex determination. A recent analysis found between 17 and 25 transitions among sex-determining mode have occurred during gecko’s long evolutionary history. However, this is most certainly a low estimate because we actually don’t know the sex-determining systems of most gecko species. In fact, we only have good data for about 50 of the 1,700 described gecko species. That’s just 3% of geckos!
It’s clear that we need to learn more about how geckos determine sex. But, how is a species’ sex-determining mode identified? Species with sex chromosomes are typically identified in one of two ways. The most obvious is to look at the chromosomes of a male and female under a light microscope, a process known as karyotyping. In some species, the X and Y (or Z and W) are different sizes and can be easily identified. Unfortunately, most species don’t have these distinct sex chromosomes but instead have sex chromosomes that are roughly the same size. Karyotyping cannot identify these sex chromosomes. Fortunately, another means of identifying sex chromosomes has emerged that uses recent advances in DNA sequencing. This process examines the DNA of multiple males and multiple females to find fragments of DNA that are unique to one sex or the other. If sex-specific DNA occurs in just male individuals this male-specific DNA is on the Y chromosome and the species has an XX/XY system. If sex-specific DNA occurs in just female individuals this female-specific DNA is on the W chromosome and the species has a ZZ/ZW system. This new method has proven incredibly useful and been used to identify sex chromosomes in a number of gecko, fish, and snake species.
Identifying whether a species has TSD is relatively simple when compared to identifying sex chromosomes. All that is needed to identify TSD are several reliable incubators and the ability to produce and raise a large number of geckos until they are old enough to be confidently sexed. Incubation experiments consist of incubating groups of eggs at different temperatures, sexing the resulting hatchlings, and then determining whether the sex ratios at each temperature treatment are statistically different than 1:1. There are numerous online statistical tools (like graphpad.com) that can calculate whether sex ratios produced at a single temperature are statistically skewed. It is worth mentioning that these sorts of experiments require a large number of individuals, typically 15-20 eggs per temperature treatment, for robust results.
The incredible diversity of gecko sex-determining systems makes them an ideal group to study how sex-determining systems evolve over time. This is an exciting area of research for evolutionary biologist that has implications for reptile hobbyists. Stay tuned as the sex-determining modes of more gecko species are uncovered and we uncover more about reproduction in these fascinating lizards.
Learn More
Bachtrog, D., J. E. Mank, C. L. Peichel, M. Kirkpatrick, S. P. Otto, T.-L. Ashman, M. W. Hahn, J. Kitano, I. Mayrose, R. Ming, N. Perrin, L. Ross, N. Valenzuela, J. C. Vamosi, and T. T. o. S. Consortium. 2014. Sex determination: Why so many ways of doing it? PLoS Biol 12:e1001899.
http://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.1001899
Ezaz, T., S. D. Sarre, D. O’Meally, J. A. M. Graves, and A. Georges. 2009. Sex chromosome evolution in lizards: Independent origins and rapid transitions. Cytogenet Genome Res 127:249–260.
http://appliedecology.edu.au/wp-content/uploads/2014/04/2010_Ezaz_Cyto_Gen_Res.pdf
Gamble, T. 2010. A review of sex determining mechanisms in geckos (Gekkota: Squamata). Sex Dev 4:88–103.
http://geckoevolution.org/publications/Gamble_2010_SD.pdf
Gamble, T. 2016. Using RAD-seq to recognize sex-specific markers and sex chromosome systems. Mol Ecol 25:2114–2116.
http://onlinelibrary.wiley.com/doi/10.1111/mec.13648/epdf
Gamble, T., T. A. Castoe, S. V. Nielsen, J. L. Banks, D. C. Card, D. R. Schield, G. W. Schuett, and W. Booth. 2017. The discovery of XY sex chromosomes in a boa and python. Curr Biol 27:2148–2153.
http://geckoevolution.org/publications/Gamble_etal_2017_CurrentBiology.pdf
Gamble, T., J. Coryell, T. Ezaz, J. Lynch, D. P. Scantlebury, and D. Zarkower. 2015. Restriction site-associated DNA sequencing (RAD-seq) reveals an extraordinary number of transitions among gecko sex-determining mechanisms. Mol Biol Evol 32:1296–1309.
http://geckoevolution.org/publications/Gamble_etal_2015_MBE.pdf
Gamble, T. and D. Zarkower. 2012. Sex determination. Curr Biol 22:R257–R262.
http://geckoevolution.org/publications/Gamble_Zarkower_2012.pdf
Gamble, T. and D. Zarkower. 2014. Identification of sex-specific molecular markers using restriction site associated DNA sequencing. Mol Ecol Resour 14:902–913.
http://geckoevolution.org/publications/Gamble_Zarkower_2014_MER.pdf
Holleley, C. E., D. O’Meally, S. D. Sarre, J. A. M. Graves, T. Ezaz, K. Matsubara, B. Azad, X. Zhang, and A. Georges. 2015. Sex reversal triggers the rapid transition from genetic to temperature-dependent sex. Nature 523:79–82.
https://www.researchgate.net/profile/Bhumika_Azad/publication/279730121_Sex_reversal_triggers_the_rapid_transition_from_genetic_to_temperature-dependent_sex/links/559c624308aee2c16df172ec/Sex-reversal-triggers-the-rapid-transition-from-genetic-to-temperature-dependent-sex.pdf
Matsubara, K., T. Gamble, Y. Matsuda, D. Zarkower, S. D. Sarre, A. Georges, J. A. M. Graves, and T. Ezaz. 2014. Non-homologous sex chromosomes in two geckos (Gekkonidae: Gekkota) with female heterogamety Cytogenet Genome Res 143:251–258.
http://geckoevolution.org/publications/Matsubara_etal_2014_CGR_Christinus.pdf
Schmid, M., C. Steinlein, T. Haaf, and A. Mijares-Urrutia. 2014. Nascent ZW Sex Chromosomes in Thecadactylus rapicauda (Reptilia, Squamata, Phyllodactylidae). Cytogenet Genome Res 143:259–267.
https://www.researchgate.net/profile/Thomas_Haaf/publication/265970867_Nascent_ZW_Sex_Chromosomes_in_Thecadactylus_rapicauda_Reptilia_Squamata_Phyllodactylidae/links/551296370cf268a4aaea90b0.pdf
Shine, R. 1999. Why is sex determined by nest temperature in many reptiles ? Trends Ecol Evol 14:186–189.
http://aerg.canberra.edu.au/library/sex_reptile/1999_Shine_TSD_review.pdf
Viets, B. E., M. A. Ewert, L. G. Talent, and C. E. Nelson. 1994. Sex–determining mechanisms in squamate reptiles. J Exp Zool 270:45–56.
http://www.lacerta.de/AS/Bibliografie/BIB_1987.pdf
Viets, B. E., A. Tousignant, M. A. Ewert, C. E. Nelson, and D. Crews. 1993. Temperature-dependent sex determination in the leopard gecko, Eublepharis macularius. J Exp Zool 265:679–683.
http://aerg.canberra.edu.au/library/sex_general/1993_Viets_etal_TSD_Eublepharis.pdf

